Hi Folks,
I'm contemplating switching my treatment system back to sodium hypochlorite from hydrogen peroxide. I'm looking for some information.
I have two problems. I have some slime producing bacteria (see this post) and I have H2S. I used to struggle when I used chlorine with occasional upsets, but that may have been my own doing as I would buy 9 to 12 months of sodium hypochlorite at a time. I know chlorine has a shelf life. How long is the maximum that I should store chlorine?
The second question is what is the impact of pH on chlorines effectiveness? My pH is 8.6 to 8.7 from two water tests.
The internet is full of charts like the following:
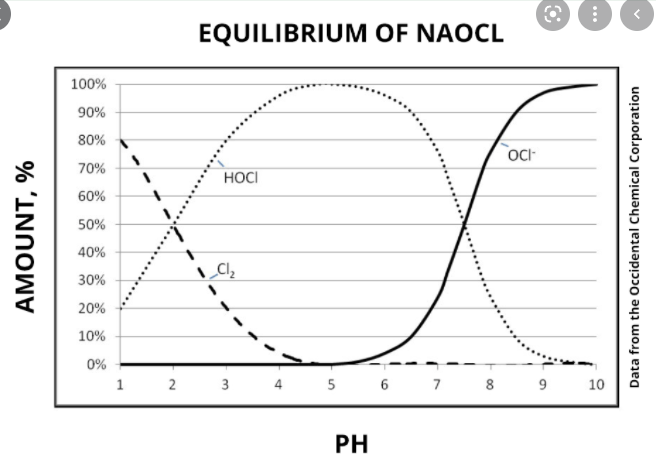
Is it the HOCl that kills bacteria or OCl? Same question for H2S. Or neither?
When I use a chlorine test kit (I use the ones for a pool where you add drops and compare shades of yellow), is it measuring the HOCl or the OCL? Or neither?
People tell me to target a residual chlorine of 0.5 to 1 ppm before going into my centaur carbon filter. I've never found that to be effective. I've always felt I have to treat at much higher residuals. Could it be the pH that is driving this, my imagination, or lack of contact time? My best test for whether the system is working is my nose and early morning showers where the contact time is >8 hours. Thus, I'm skeptical contact time is the issue, but I thought I would ask. I have a 80 gallon contact tank.
Thank you
I'm contemplating switching my treatment system back to sodium hypochlorite from hydrogen peroxide. I'm looking for some information.
I have two problems. I have some slime producing bacteria (see this post) and I have H2S. I used to struggle when I used chlorine with occasional upsets, but that may have been my own doing as I would buy 9 to 12 months of sodium hypochlorite at a time. I know chlorine has a shelf life. How long is the maximum that I should store chlorine?
The second question is what is the impact of pH on chlorines effectiveness? My pH is 8.6 to 8.7 from two water tests.
The internet is full of charts like the following:
Is it the HOCl that kills bacteria or OCl? Same question for H2S. Or neither?
When I use a chlorine test kit (I use the ones for a pool where you add drops and compare shades of yellow), is it measuring the HOCl or the OCL? Or neither?
People tell me to target a residual chlorine of 0.5 to 1 ppm before going into my centaur carbon filter. I've never found that to be effective. I've always felt I have to treat at much higher residuals. Could it be the pH that is driving this, my imagination, or lack of contact time? My best test for whether the system is working is my nose and early morning showers where the contact time is >8 hours. Thus, I'm skeptical contact time is the issue, but I thought I would ask. I have a 80 gallon contact tank.
Thank you
