FredWurlitzer
New Member
So I finally got around to getting my well water test completed and sent in to NTL. This is from a hose bib in the basement before the treatment system. Here are the results:
Total coliform and E.coli were absent
Aluminum - ND (not detected)
Arsenic - ND
Barium - ND
Cadmium - ND
Calcium - 177.3 mg/L
Chromium - ND
Copper - 0.087 mg/L
Iron - 6.444 mg/L
Lead - 0.003 mg/L
Lithium - 0.020 mg/L
Magnesium - 87.38 mg/L
Manganese 0.067 mg/L
Mercury - ND
Nickel - ND
Potassium - 4.1 mg/L
Selenium - ND
Silica - 16.4 mg/L
Silver - ND
Sodium - 393 mg/L
Strontium - 0.326 mg/L
Uranium - 0.002 mg/L
Zinc - 0.337 mg/L
Alkalinity (total as CaCO3) - 380 mg/L
Hardness - 800 mg/L
pH - 7.2
Total dissolved solids - 1,700 mg/L
Turbidity - 92 NTU
Bromide - ND
Chloride - 760 mg/L
Fluoride - ND
Nitrate - ND
Nitrite - ND
Ortho phosphate - ND
Sulfate - 62.0 mg/L
So this leads me to wonder if my current treatment setup is sufficient and/or efficient enough to handle our well water. Not sure if any of you remember my previous post from months ago but I currently have:
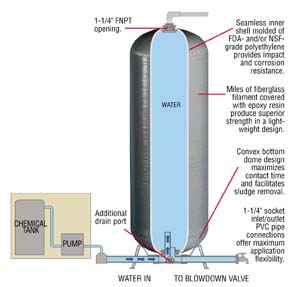
Line from well --> "degasser" or reservoir --> pressure pump --> 60 gallon fiberglass contact tank with chlorine injection --> Culligan "water conditioner" (rebed in 2015) --> Culligan water softener (rebed in 2015)
3 people in the house, we don't drink the water but do have an under the sink RO system. I clearly have a lot of iron, and it seems to be getting through the system, as I can see it build up in the toilet bowl. I'm also seeing hardness deposits which makes me question if the softener is working or set up correctly. If my math is right, 800 mg/L of hardness is about 46-47 grains per gallon, very hard.
I feel the "degasser" or large holding tank (holds at least 200 gallons) presents an interesting situation. This vessel is technically open to air, vents outside, and is not under pressure leading to iron oxidation. When the house pressure goes down, it kicks the pressure pump on which transfers that oxidized/ferric iron from the degasser/reservoir into the contact tank. The degasser also contains a float inside which activates the well pump when it gets low. I have no idea what my well pump flow rate is. I wouldn't mind ditching the Culligan stuff if need be, as it is outrageously expensive. So if you're still with me, here are my questions:
- If I fill were to fill a white bucket with cold tap water, there is slight murky tint to it. Shouldn't the Culligan water conditioner be filtering out the ferric iron and/or sediment after the contact tank? It would be nice to fill my chlorine solution tank with tap water and not my RO water.
(I'm not sure what media is in the conditioner)
-Between oxidizing the iron in the degasser, chlorine contact tank and the water softener, how is iron still getting through and staining?
-What should my 12.5% chlorine solution be dosed at?
-I know how to access the settings on my softener, but not sure if the regeneration time/limit is accurate based on my hardness and usage. I figure 3 people at 75 gal/day is 225 gals/day. Multiply that by the iron content and 46 gpg of hardness and I get 14,400 grains per day that needs to be removed. I have no idea if my softener can handle that.
Total coliform and E.coli were absent
Aluminum - ND (not detected)
Arsenic - ND
Barium - ND
Cadmium - ND
Calcium - 177.3 mg/L
Chromium - ND
Copper - 0.087 mg/L
Iron - 6.444 mg/L
Lead - 0.003 mg/L
Lithium - 0.020 mg/L
Magnesium - 87.38 mg/L
Manganese 0.067 mg/L
Mercury - ND
Nickel - ND
Potassium - 4.1 mg/L
Selenium - ND
Silica - 16.4 mg/L
Silver - ND
Sodium - 393 mg/L
Strontium - 0.326 mg/L
Uranium - 0.002 mg/L
Zinc - 0.337 mg/L
Alkalinity (total as CaCO3) - 380 mg/L
Hardness - 800 mg/L
pH - 7.2
Total dissolved solids - 1,700 mg/L
Turbidity - 92 NTU
Bromide - ND
Chloride - 760 mg/L
Fluoride - ND
Nitrate - ND
Nitrite - ND
Ortho phosphate - ND
Sulfate - 62.0 mg/L
So this leads me to wonder if my current treatment setup is sufficient and/or efficient enough to handle our well water. Not sure if any of you remember my previous post from months ago but I currently have:
Line from well --> "degasser" or reservoir --> pressure pump --> 60 gallon fiberglass contact tank with chlorine injection --> Culligan "water conditioner" (rebed in 2015) --> Culligan water softener (rebed in 2015)
3 people in the house, we don't drink the water but do have an under the sink RO system. I clearly have a lot of iron, and it seems to be getting through the system, as I can see it build up in the toilet bowl. I'm also seeing hardness deposits which makes me question if the softener is working or set up correctly. If my math is right, 800 mg/L of hardness is about 46-47 grains per gallon, very hard.
I feel the "degasser" or large holding tank (holds at least 200 gallons) presents an interesting situation. This vessel is technically open to air, vents outside, and is not under pressure leading to iron oxidation. When the house pressure goes down, it kicks the pressure pump on which transfers that oxidized/ferric iron from the degasser/reservoir into the contact tank. The degasser also contains a float inside which activates the well pump when it gets low. I have no idea what my well pump flow rate is. I wouldn't mind ditching the Culligan stuff if need be, as it is outrageously expensive. So if you're still with me, here are my questions:
- If I fill were to fill a white bucket with cold tap water, there is slight murky tint to it. Shouldn't the Culligan water conditioner be filtering out the ferric iron and/or sediment after the contact tank? It would be nice to fill my chlorine solution tank with tap water and not my RO water.
(I'm not sure what media is in the conditioner)
-Between oxidizing the iron in the degasser, chlorine contact tank and the water softener, how is iron still getting through and staining?
-What should my 12.5% chlorine solution be dosed at?
-I know how to access the settings on my softener, but not sure if the regeneration time/limit is accurate based on my hardness and usage. I figure 3 people at 75 gal/day is 225 gals/day. Multiply that by the iron content and 46 gpg of hardness and I get 14,400 grains per day that needs to be removed. I have no idea if my softener can handle that.