Jaylivi
Member
Good mornning,
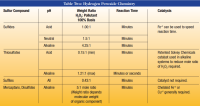
I finally installed a softener and KL backwashing system with hydrogen peroxide injection. The tank for the hydrogen peroxide solution is quite large and I believe I need to mix it with water, but how do I know how much water to use with one gallon of nuetra sul 7% solution?
My water:
ph 7.8
H 400 mg/l
iron 2.61
sulfate 52.8
nitrate .15
So, do I dilute the 7% solution in the holding tank? Also, what is the easiest way to change how much I soften the water?
Thanks,
Jay
I finally installed a softener and KL backwashing system with hydrogen peroxide injection. The tank for the hydrogen peroxide solution is quite large and I believe I need to mix it with water, but how do I know how much water to use with one gallon of nuetra sul 7% solution?
My water:
ph 7.8
H 400 mg/l
iron 2.61
sulfate 52.8
nitrate .15
So, do I dilute the 7% solution in the holding tank? Also, what is the easiest way to change how much I soften the water?
Thanks,
Jay