ChadWright
New Member
Hello..this may be long  as i have been looking or a forum like this for a LONG time...I live in East Central Illinois, on a well, and have some pretty bad water...Small town with no one that really knows anything about proper water treatment, unless you consider Culligan that :-(... Long story short...I ended up going with a company online "Crystal Quest Water Systems" that i thought would help me with my issues...We spoke/emailed back and forth a lot...I sent them my water analysis etc...My biggest complaints with my water is, Iron and high Arsenic levels...I ended up going with a system that they recommended that has a 20" sediment filter, then 2 tanks with fleck 5600sxt heads ( one is their whole house filtration tank, the other is the arsenic removal tank) then it runs through another 20" solid carbon cartridge...I have my water softener in line after the first 20" sediment filter..and a POU RO system under the kitchen sink with a 98% rejection DOW membrane..Once hooked up, everything worked great...water was clear and clean, and after sending a sample away the arsenic levels were 0...this was all pretty short lived...my water gradually turned back to looking pretty dingy when a bathtub was ran, and the arsenic levels started to creep up again...I feel like i was just sold what they would make the best bang for their buck on...after doing more research, i think i can come up with my own system with the professionals help here...I will attach a screen print of my water analysis from the Illinois Water Survey and will also give you a snippit of what i currently have hooked up now...I am hoping with suggestions from you all i can get back to clear/clean water...I am hoping to be able to use the heads/tanks etc that i currently have and make a media change or additions as needed...I have been reading up on the Katalox Light media and it seems like it may fit the bill for what i am needing...I am fine to do this in stages etc...Please let me know what suggestions you may have..
as i have been looking or a forum like this for a LONG time...I live in East Central Illinois, on a well, and have some pretty bad water...Small town with no one that really knows anything about proper water treatment, unless you consider Culligan that :-(... Long story short...I ended up going with a company online "Crystal Quest Water Systems" that i thought would help me with my issues...We spoke/emailed back and forth a lot...I sent them my water analysis etc...My biggest complaints with my water is, Iron and high Arsenic levels...I ended up going with a system that they recommended that has a 20" sediment filter, then 2 tanks with fleck 5600sxt heads ( one is their whole house filtration tank, the other is the arsenic removal tank) then it runs through another 20" solid carbon cartridge...I have my water softener in line after the first 20" sediment filter..and a POU RO system under the kitchen sink with a 98% rejection DOW membrane..Once hooked up, everything worked great...water was clear and clean, and after sending a sample away the arsenic levels were 0...this was all pretty short lived...my water gradually turned back to looking pretty dingy when a bathtub was ran, and the arsenic levels started to creep up again...I feel like i was just sold what they would make the best bang for their buck on...after doing more research, i think i can come up with my own system with the professionals help here...I will attach a screen print of my water analysis from the Illinois Water Survey and will also give you a snippit of what i currently have hooked up now...I am hoping with suggestions from you all i can get back to clear/clean water...I am hoping to be able to use the heads/tanks etc that i currently have and make a media change or additions as needed...I have been reading up on the Katalox Light media and it seems like it may fit the bill for what i am needing...I am fine to do this in stages etc...Please let me know what suggestions you may have..
Here is the system that i currently have in place:
http://www.crystalquest.com/how arsenic water filters work.htm
I have attached my water report..this is untreated water from a yard hydrant:
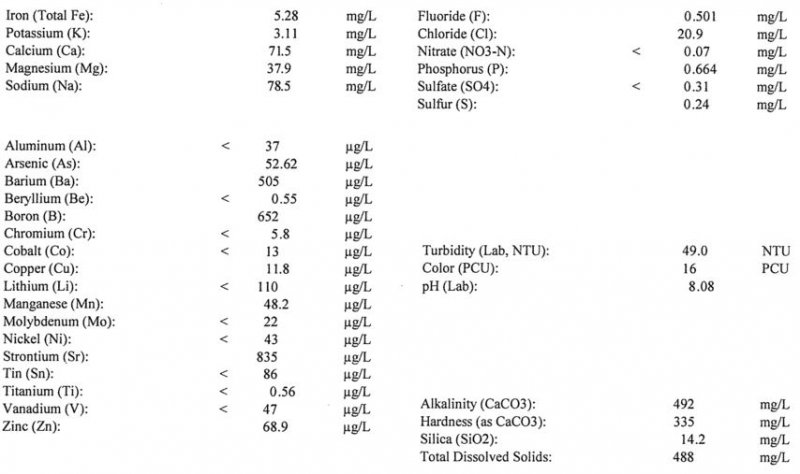
Thank you,
Chad Wright
Here is the system that i currently have in place:
http://www.crystalquest.com/how arsenic water filters work.htm
I have attached my water report..this is untreated water from a yard hydrant:
Thank you,
Chad Wright
