It is an incorrect practice to dilute sodium hypochlorite bleach with water, unless steps are taken to adjust the pH of the diluted bleach to the original pH. Failure to do so, and to maintain the temperature of bleach (diluted or undiluted) below 50° F. will lead to the rapid breakdown of sodium hypochlorite into other compounds, some of which can be harmful after certain concentrations are reached.
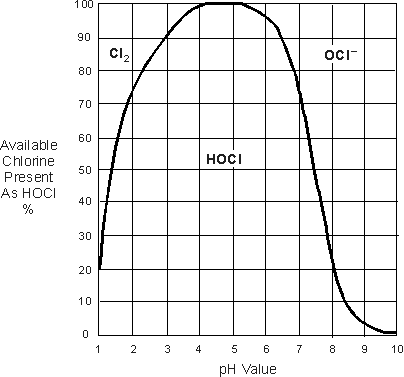
Sodium hypochlorite bleach is preserved by the addition of the base sodium hydroxide, which maintains the pH of the bleach at over 12. When water, usually at a pH of 6-8, is added to bleach, the pH of the bleach is automatically lowered. Once the pH of the bleach solution is below 11.86, some of the hypochlorite (NaOCl or OCl-) is transformed into hypochlorous acid (HOCl) and the balance between the two is directly dependent on the pH, as this diagram shows:
Hypochlorous acid (HOCl) is a powerful oxidiser, as its oxygen atom is easily lost. It is this oxidising action that allows it to kill bacteria, by entering the cell wall of the bacteria and destroying DNA. However, when it does lose this single oxygen atom, it leaves behind only a single hydrogen and chlorine atom, or HCl, which everyone knows is hydrochloric acid.
So, if one dilutes bleach with water 1:5 and ends up with a solution with a pH of (just a guess) 8, he would have a bleach solution that was 80% sodium hypochlorite, and 20% hypochlorous acid. As the hypochlorous acid deteriorates to form the stronger hypochloric acid, the pH of the solution is steadily lowered by the creation of HCL. Steadily lowering the pH to, let's say, 7, will now make hypochlorous acid the predominant factor in a 70/30% split, as seen above. Now, the greater volume of hypochlorous acid loses even more oxygen and causes even more HCl to form, lowering the pH further. At a point roughly between 4 and 5 pH, as seen in the diagram, free chlorine gas will begin to leave the solution.
This process can be retarded somewhat by keeping the stored solution refrigerated. It is also naturally slowed somewhat by the very low vapour pressure of hypochlorous acid, meaning that a large vat of diluted bleach only oxidises from the upper layer, although it will mix by itself over time.
The Chlorine Institute of America has very good literature on their website about the proper handling and storage of bleach. One of their articles deals with the proper method of diluting bleach with water. It involves the very careful addition of sodium hydroxide (NaOH) to diluted bleach to bring the pH back up over 12, and the use of a good pH tester to tell when this objective is reached.