I think that's all it takes, but you could use a teaspoon to be really sure.. So if I understand correctly, a few drops of bleach should precipitate out some iron?
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Is this ferrous iron or iron bacteria? Does it create wispies if allowed to settle?
- Thread starter Dis360
- Start date
Users who are viewing this thread
Total: 3 (members: 0, guests: 3)
Dis360
Member
To get a better idea of what is in my water I put this quick video together:
https://drive.google.com/file/d/1_CZIvAnRrPnd6X00jPFdLZl6tM9bHHcI/view?usp=sharing
https://drive.google.com/file/d/1_CZIvAnRrPnd6X00jPFdLZl6tM9bHHcI/view?usp=sharing
Sponsor
Paid Advertisement
Dis360
Member
If it walks like iron and quacks like iron it must be.... well you know.
I added a teaspoon of bleach to a water sample, this is it after ~24 hours. Fluffy rust colored deposits.

I knew there had to be iron in this water, I decided to purchase a different brand of water testing strips. Look at this ~4ppm of iron, I confirmed this with a second test.
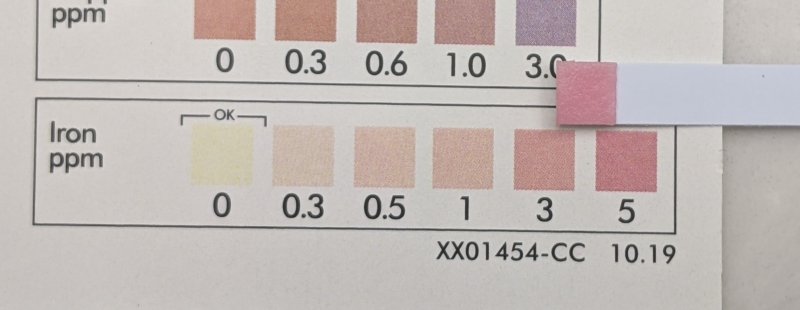
I've learned a lesson here, don't trust water test strips. I am going to get a real lab test at this point. I believe I have a fair amount of ferrous iron in my water supply and it's also turning into iron bacteria and that's what shows up in the bottom of a water sample after ~24 hours.
My plan moving forward is to get a lab water test, then shock my well with chlorine to kill as much iron bacteria possible, then treat iron bacteria/ferrous iron initially with a KDF filter to start eliminating it immediately, then as a permit solution install an ozone filter tank like an iron curtain storm or similar.
Daniel
I added a teaspoon of bleach to a water sample, this is it after ~24 hours. Fluffy rust colored deposits.
I knew there had to be iron in this water, I decided to purchase a different brand of water testing strips. Look at this ~4ppm of iron, I confirmed this with a second test.
I've learned a lesson here, don't trust water test strips. I am going to get a real lab test at this point. I believe I have a fair amount of ferrous iron in my water supply and it's also turning into iron bacteria and that's what shows up in the bottom of a water sample after ~24 hours.
My plan moving forward is to get a lab water test, then shock my well with chlorine to kill as much iron bacteria possible, then treat iron bacteria/ferrous iron initially with a KDF filter to start eliminating it immediately, then as a permit solution install an ozone filter tank like an iron curtain storm or similar.
Daniel
Ĝan Ŭesli Starling
Member
Have you, perhaps, iron pipes? A well-water test is performed on water collected after leaving water run long enough to flush out the system. So that the water to be tested is fresh from the well, not left sit all night inside the expansion tank.
Should you have corroding iron pipes, then the test won't have shown that too very well (because of the flush-out). And so far as I know (no expert I) the NTL report on bacteria targets only the harmful sort, coliform and its ilk, not any harmless-to-humans bacteria. Anyhow, that's what mine showed. An absence of E. Coli, etc. Line items specifically showing the absence of a long list of things. No line item at all for iron bacteria. Yet it is certain that I do have iron bacteria. Everyone in my region does.
The pre-test flush-out may likely have scrubbed away any truly loose iron corrosion along with any poorly adhering iron bacteria. Then afterward, when left to sit, the colony repopulates. Just an amateur's hypothesis.
Check the plastic flapper valve inside your toilet tank. Both the reducing and oxidizing types of iron bacteria adhere well to plastic. If it feels slimy, that's a pretty strong clue. My own iron level is only 1.2 ppm, and mine feels like that. My own water does somewhat like yours. Clear at first, but with feathery growth at the bottom over time. Only just slightly orange, that growth. More orange yet inside my shower.
Also my taps, when first turned on after having been off all night, give out a very brief whiff of H2S. That's from the water trapped above the turn off valve, but still inside the spigot, enjoying lots of oxygen all night long. So likely I have sulfate-reducing bacteria too. Those, so I read, co-occur often with iron bacteria.
Should you have corroding iron pipes, then the test won't have shown that too very well (because of the flush-out). And so far as I know (no expert I) the NTL report on bacteria targets only the harmful sort, coliform and its ilk, not any harmless-to-humans bacteria. Anyhow, that's what mine showed. An absence of E. Coli, etc. Line items specifically showing the absence of a long list of things. No line item at all for iron bacteria. Yet it is certain that I do have iron bacteria. Everyone in my region does.
The pre-test flush-out may likely have scrubbed away any truly loose iron corrosion along with any poorly adhering iron bacteria. Then afterward, when left to sit, the colony repopulates. Just an amateur's hypothesis.
Check the plastic flapper valve inside your toilet tank. Both the reducing and oxidizing types of iron bacteria adhere well to plastic. If it feels slimy, that's a pretty strong clue. My own iron level is only 1.2 ppm, and mine feels like that. My own water does somewhat like yours. Clear at first, but with feathery growth at the bottom over time. Only just slightly orange, that growth. More orange yet inside my shower.
Also my taps, when first turned on after having been off all night, give out a very brief whiff of H2S. That's from the water trapped above the turn off valve, but still inside the spigot, enjoying lots of oxygen all night long. So likely I have sulfate-reducing bacteria too. Those, so I read, co-occur often with iron bacteria.
Last edited:
Ĝan Ŭesli Starling
Member
My mistake, you said KDF filter, not media. So ignore all below. I'd delete it, if I knew how.
I'd like to use KDF media myself, it being largely self-immune to colonization by bacteria owing to mild electric currents. But it's way heavy. Doesn't lift and swirl very easy. So takes a lot of flow to regenerate, on the order of 30gpm per square foot of bed surface area.
My well-system measured out at only 12gpm. I'm wanting to go with Vortech tanks. And those don't come any smaller than 9x48. And one chart gives a backwash rate of 13.3gpm for KDF, versus 4gpm for Katalox Lite. And in any case, I wouldn't want that high a backwash no matter what.
I'd like to use KDF media myself, it being largely self-immune to colonization by bacteria owing to mild electric currents. But it's way heavy. Doesn't lift and swirl very easy. So takes a lot of flow to regenerate, on the order of 30gpm per square foot of bed surface area.
My well-system measured out at only 12gpm. I'm wanting to go with Vortech tanks. And those don't come any smaller than 9x48. And one chart gives a backwash rate of 13.3gpm for KDF, versus 4gpm for Katalox Lite. And in any case, I wouldn't want that high a backwash no matter what.
Last edited:
There is an Edit button.My mistake, you said KDF filter, not media. So ignore all below. I'd delete it, if I knew how.
ditttohead
Water systems designer, R&D
That is not what KDF is for. You need to get a real water test done. Once the test is completed we can come up with a solution for you. As to the iron bacteria, you need to sanitize the well and all of your plumbing. There are many treatment possibilities but without a well test, we can only throw out guesses.
NTLWATERTEST
use the link above, look for well standard. This particular company has been great to work with for many years.
NTLWATERTEST
use the link above, look for well standard. This particular company has been great to work with for many years.
Dis360
Member
Have you, perhaps, iron pipes? A well-water test is performed on water collected after leaving water run long enough to flush out the system. So that the water to be tested is fresh from the well, not left sit all night inside the expansion tank.
I did allow my pressure tank to cycle a couple of times before collecting the sample. My house was built in 1993 and all the pipes in the house are polybutylene and PEX with copper and brass fitting. Now I don't know the makeup of the pipe underground from the well to the house, although the pipe that I can see above ground at the well and near the pressure tank is not metal. I plan on collecting a sample at the well head for comparison.
I did another iron strip test, one made by Sensafe called Ida's Iron, comes highly recommended.
This specifically tests for FE+2 (ferrous iron), I confirmed the amount I found before, ~3ppm -- ~4ppm
I then tested water from my fridge water filter, which is a 0.5 micron cocoanut carbon block and, check it out, it's able to remove the ferrous iron with micro filtration.
I am fairly certain what I have going on here is, ferrous iron in my well water that never really touches much oxygen, then once the water escapes from the taps, it forms into iron bacteria, or the second scenario, I have ferrous iron and iron bacteria in my well and when the water escapes from the taps it multiplies into more iron bacteria with the introduction of oxygen. I am going to try adding a 1 micron whole house filter to my existing setup and see if that's small enough to remove the Fe+2.
I will still be getting a lab water test but if anyone needs to test for Fe+2 I highly recommend the brand mentioned above (amazon).
Last edited:
LLigetfa
DIYer, not in the trades
Actually the exposure to oxygen converts ferrous to ferric. That is the premise under which some iron filters work. Ferric iron will precipitate so that it can be mechanically filtered out. Oxygen (air) is sometimes used but there are other means such as chlorine.I am fairly certain what I have going on here is, ferrous iron in my well water that never really touches much oxygen, then once the water escapes from the taps, it forms into iron bacteria
Iron bacteria is not the product of ferrous or ferric iron. Iron bacteria consumes iron and leaves a residue.
Dis360
Member
Actually the exposure to oxygen converts ferrous to ferric. That is the premise under which some iron filters work. Ferric iron will precipitate so that it can be mechanically filtered out. Oxygen (air) is sometimes used but there are other means such as chlorine.
Iron bacteria is not the product of ferrous or ferric iron. Iron bacteria consumes iron and leaves a residue.
From what I understand iron bacteria can convert in both directions, not sure about the oxygen requirement for each, as I understand IOB needs O2 and IRB doesn't as it uses other sources, like light or other organic compounds.
Reference:
https://onlinelibrary.wiley.com/doi/abs/10.1002/047147844X.wq406
"Two types of iron bacteria (iron‐oxidizing bacteria and iron‐reducing bacteria) are described in this article. Iron‐oxidizing bacteria get energy through the oxidation of ferrous (Fe2+) to ferric (Fe3+) in aerobic condition, which can cause undesirable odors, tastes, and corrosion in well systems and water distribution systems. Moreover, they are the major role in the formation of acid mine drainage, which finally leads to acid creeks in mining regions. Iron‐reducing bacteria convert ferric (Fe3+) to ferrous (Fe2+) under anoxic condition with the presence of organic compounds as electron donor"
IOB = ferrous --> ferric (aerobic condition), it OXIDIZES Fe(II) to Fe(III)
IRB = ferric --> ferrous (anoxic condition), it REDUCES Fe(III) to Fe(II)
From what I've researched regarding oxygenation tanks it converts ferrous to ferric iron then filters out the precipitate, I assume that's appropriate for IOB. If an oxygenation tank is used when IRB is present it will have a reverse effect and will increase the bacteria, which makes sense as it's feeding it, therefore something like an ozonating (O3) tank will precipitate ferric iron from IRB.
I think I have IOB, since IRB would have Fe(III) in it as it comes out of the tap and wouldn't be clear while IOB would be clear as it contains Fe(II) (clear water iron). Is it safe to say there is ferric iron in the well being reduced to ferrous iron by iron bacteria? By the time it makes it out of the taps more oxygen is introduced, then more ferrous iron is oxidized to ferric leaving slime everywhere or it could be the other way around if I do really have IRB and Fe3 would be converted to Fe2 with the introduction of oxygen.
Is it also safe to say you can have IOB/IRB but no iron for it to feed on or is iron required for the bacteria to even exist? Perhaps like my 0.5 micron filter, filtering out ferric/ferrous iron leaving the iron bacteria nothing to feed on (if its even able to make it through the filter)?
Last edited:
LLigetfa
DIYer, not in the trades
What I was trying to say is that the presence of iron does not produce the bacteria out of nothing. The bacteria has to already be present and simply feeds* on the iron, be it aerobic or anoxic. If your water starts out clear and goes cloudy after exposure to air, it is a ferrous to ferric conversion. If you have evidence of bacteria, it could be IOB or IRB.
*Feeds - initially I said consumes. You said converts, oxidizes, reduces.
The bacteria doesn't work for free. It derives a benefit from the work it does which sustains and produces more bacteria.
*Feeds - initially I said consumes. You said converts, oxidizes, reduces.
The bacteria doesn't work for free. It derives a benefit from the work it does which sustains and produces more bacteria.
Gsmith22
Active Member
This is painful watching this play out. OP, please get a real well test done that is comprehensive - NTL was recommended above and is fine. I've used a local county cooperative who partnered with testing agencies to do the same thing. Using NTL as an example, if you have never had any well test done than I would recommend their "Deluxe" well water test and then be prepared to do a "Basic"+specific test for whatever specific condition you have (such as Iron, Gross Alpha, Lead, etc.) at least once a year. I did a "deluxe" type test on my first test and was shocked at what was in the well water and it changed how treatment was going to be done. Its typically not one condition, its usually several problems and depending on the combination, one media might be preferred over another. Just focusing on iron isn't enough even though that might be what you can visually see as problematic.
For a specific problem like ferrous or ferric iron, if you desire to monitor that more frequently than a yearly lab test, then ditch the test strips and get a titration test kit for that specifically. LaMotte, Hatch or Taylor are well known brands that make titration kits. Outside of a lab, a titration kit is your best bet if you want to monitor something more regularly, be accurate, and not blow a hole in your wallet. There are certainly more expensive options. Test strips are not substitutes for any of what I mention above. Simply put, they are garbage not worth the paper they are printed on. I have both a well and pool and have been down this road attempting to quantify what is going on with water chemistry via test strips. I'll save you the hassle, test strips won't help you out and can literally disguise what is going on because they can be so inaccurate.
OP, neither you or anyone on here can help figure out what to do without knowledge of what is going on in the water and test strips won't provide that knowledge.
For a specific problem like ferrous or ferric iron, if you desire to monitor that more frequently than a yearly lab test, then ditch the test strips and get a titration test kit for that specifically. LaMotte, Hatch or Taylor are well known brands that make titration kits. Outside of a lab, a titration kit is your best bet if you want to monitor something more regularly, be accurate, and not blow a hole in your wallet. There are certainly more expensive options. Test strips are not substitutes for any of what I mention above. Simply put, they are garbage not worth the paper they are printed on. I have both a well and pool and have been down this road attempting to quantify what is going on with water chemistry via test strips. I'll save you the hassle, test strips won't help you out and can literally disguise what is going on because they can be so inaccurate.
OP, neither you or anyone on here can help figure out what to do without knowledge of what is going on in the water and test strips won't provide that knowledge.
IOB is the more rare of the two. It requires an oxygen rich environment. Wells and water heaters are generally oxygen deficient, so IRB is the most prevalent. ferric is easily removed by mechanical filtration, while ferrous requires oxidation to convert to ferric, then (again) remove by filtration.From what I understand iron bacteria can convert in both directions, not sure about the oxygen requirement for each, as I understand IOB needs O2 and IRB doesn't as it uses other sources, like light or other organic compounds.
Reference:
https://onlinelibrary.wiley.com/doi/abs/10.1002/047147844X.wq406
"Two types of iron bacteria (iron‐oxidizing bacteria and iron‐reducing bacteria) are described in this article. Iron‐oxidizing bacteria get energy through the oxidation of ferrous (Fe2+) to ferric (Fe3+) in aerobic condition, which can cause undesirable odors, tastes, and corrosion in well systems and water distribution systems. Moreover, they are the major role in the formation of acid mine drainage, which finally leads to acid creeks in mining regions. Iron‐reducing bacteria convert ferric (Fe3+) to ferrous (Fe2+) under anoxic condition with the presence of organic compounds as electron donor"
IOB = ferrous --> ferric (aerobic condition), it OXIDIZES Fe(II) to Fe(III)
IRB = ferric --> ferrous (anoxic condition), it REDUCES Fe(III) to Fe(II)
From what I've researched regarding oxygenation tanks it converts ferrous to ferric iron then filters out the precipitate, I assume that's appropriate for IOB. If an oxygenation tank is used when IRB is present it will have a reverse effect and will increase the bacteria, which makes sense as it's feeding it, therefore something like an ozonating (O3) tank will precipitate ferric iron from IRB.
I think I have IOB, since IRB would have Fe(III) in it as it comes out of the tap and wouldn't be clear while IOB would be clear as it contains Fe(II) (clear water iron). Is it safe to say there is ferric iron in the well being reduced to ferrous iron by iron bacteria? By the time it makes it out of the taps more oxygen is introduced, then more ferrous iron is oxidized to ferric leaving slime everywhere or it could be the other way around if I do really have IRB and Fe3 would be converted to Fe2 with the introduction of oxygen.
Is it also safe to say you can have IOB/IRB but no iron for it to feed on or is iron required for the bacteria to even exist? Perhaps like my 0.5 micron filter, filtering out ferric/ferrous iron leaving the iron bacteria nothing to feed on (if its even able to make it through the filter)?
Bannerman
Well-Known Member
While IRB is not harmful if consumed, it is not a bacteria desired by most. To eliminate it, a sanitizer such as chlorine will be typically utilized which will kill the bacteria, with an added benefit of oxidizing ferrous iron, converting it to ferric which will be removed by the KL media.
When operating a private well, you become your own municipality. You are responsible for both treatment and safety. To properly address water issues, you first need to understand the composition of the water. Some conditions may seem unimportant or irrelavent but those can sometimes effect which treatment methods are needed for the conditions that are considered a problem. A comprehensive lab analysis is required initially, and as water conditions can change, further periodic testing will indicate if changes have occured.
When operating a private well, you become your own municipality. You are responsible for both treatment and safety. To properly address water issues, you first need to understand the composition of the water. Some conditions may seem unimportant or irrelavent but those can sometimes effect which treatment methods are needed for the conditions that are considered a problem. A comprehensive lab analysis is required initially, and as water conditions can change, further periodic testing will indicate if changes have occured.
Dis360
Member
This is painful watching this play out. OP, please get a real well test done that is comprehensive - NTL was recommended above and is fine.
It's the opposite of painful for me. I truly do appreciate the effort to inspire me to get a lab test and I will be doing so but a lot of this is for me to gain more knowledge. I wouldn't invest or attempt to resolve a problem with water condition that I do know the source of. I've learned a lot just picking everyone's brain more knowledgeable than me. I've also learned a lot of lab water tests don't include an iron bacteria test, I would have probably paid a couple hundred dollars and only then realized I have ferrous iron in my water but still have iron bacteria slime everywhere.
Dis360
Member
IOB is the more rare of the two. It requires an oxygen rich environment. Wells and water heaters are generally oxygen deficient, so IRB is the most prevalent. ferric is easily removed by mechanical filtration, while ferrous requires oxidation to convert to ferric, then (again) remove by filtration.
Ok that makes sense, I originally thought it was IRB, then started getting information overload and reading IRB occurs in lack of oxygen and IOB the opposite and thought it might be IOB since oxygen hits the water coming out of the taps and slowly turns water hazy and creates deposits in the bottom of the sample. I'm back to thinking IRB.
Dis360
Member
While IRB is not harmful if consumed, it is not a bacteria desired by most. To eliminate it, a sanitizer such as chlorine will be typically utilized which will kill the bacteria, with an added benefit of oxidizing ferrous iron, converting it to ferric which will be removed by the KL media.
That's good advice, I have sanitized my well twice over the past 9 months and each time appears to reduce the amount of iron bacteria inside the house but I suppose this requires continuous maintenance as the bacteria is being introduced into the well and will always come back.
this is the best advice. you first have to know what you're treating for. I understand your desire to do research to understand things better, but sometimes it's best left to the professionals, as they have the knowledge and experience that's required to obtain the proper treatment method.While IRB is not harmful if consumed, it is not a bacteria desired by most. To eliminate it, a sanitizer such as chlorine will be typically utilized which will kill the bacteria, with an added benefit of oxidizing ferrous iron, converting it to ferric which will be removed by the KL media.
When operating a private well, you become your own municipality. You are responsible for both treatment and safety. To properly address water issues, you first need to understand the composition of the water. Some conditions may seem unimportant or irrelavent but those can sometimes effect which treatment methods are needed for the conditions that are considered a problem. A comprehensive lab analysis is required initially, and as water conditions can change, further periodic testing will indicate if changes have occured.
Gsmith22
Active Member
It's the opposite of painful for me. I truly do appreciate the effort to inspire me to get a lab test and I will be doing so but a lot of this is for me to gain more knowledge. I wouldn't invest or attempt to resolve a problem with water condition that I do know the source of. I've learned a lot just picking everyone's brain more knowledgeable than me. I've also learned a lot of lab water tests don't include an iron bacteria test, I would have probably paid a couple hundred dollars and only then realized I have ferrous iron in my water but still have iron bacteria slime everywhere.
Your focused on iron because that is what you can see. most harmful things aren't detected by your eyes which is why a comprehensive test is needed. Iron may turn out to be a nuisance and not the real problem. More importantly, the composition of your water determines how you treat something. you may end up with an entirely different treatment method because of what is dissolved even if the only thing you are treating is "iron". If you only spend several hundred dollars on initial well testing, you are probably spending too little. this is what comes with being your own water municipality.
FruitfulPanda
Member
That's good advice, I have sanitized my well twice over the past 9 months and each time appears to reduce the amount of iron bacteria inside the house but I suppose this requires continuous maintenance as the bacteria is being introduced into the well and will always come back.
Edit: The below is not absolute. It depends on the kind of iron bacteria and iron reducing bacteria is far more common and naturally occurring. See Ĝan Ŭesli Starling's reply immediately after this.
Actually this shouldn't be the case. I've had my own battles with iron bacteria and my research indicates it mostly becomes introduced to a well when work is done like if the pump is replaced or some other maintenance, or if the well isn't sealed with a good cap. I haven't tested for it but can tell since my water went from coming out pretty orange to virtually clear since the installers shocked the well after replacing the pump a few months back.
Last edited:
Similar threads
- Replies
- 3
- Views
- 168
- Replies
- 9
- Views
- 704
- Replies
- 0
- Views
- 186
- Replies
- 3
- Views
- 465
